Anchoring autoimmune T cells to tumors by antibody-bridges
to increase the safety and efficacy of checkpoint inhibitors
SUMMARY
The Nobel-prize awarded checkpoint inhibitors that manipulate the immune system started a Cancer Immunotherapy Revolution. This resulted in regression of cancer in a minority of patients, while the majority suffered severe immune-related adverse events (irAEs) due to tolerance breakdown. Exploiting the well-known antibody-based pretargeting technology we invented a method that anchor autoimmune T cells to tumors such that healthy organs are spared. The pretargeting technology could easily be developed into an off-the-shelve product.
PROBLEM
Hundreds of clinical trials are under way with the newly approved checkpoint inhibitors that manipulate T cells to attack tumors. While the number of terminal cancer patients achieving complete remissions are increasing, immune cells unleashed by checkpoint blockades attack healthy, vital organs. Incidence of iatrogenic immune-related adverse events (IrAEs) may reach up to 90% of patients and can affect any tissue severly restricting the indications of the new drugs.
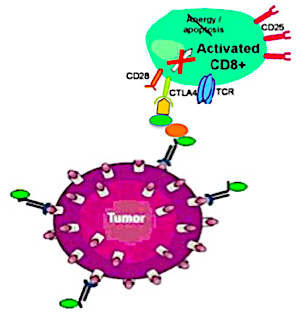
SOLUTION
-
Harnessing unleashed T cells by pretargeting autoimmunity could be turned from the Achilles' heel of immunotherapy into a powerful anticancer force
-
This technology is capable to target all types of cancer that carry Tumor Associated Antigens (TAA)
-
Since many anti-CTLA-4 antibodies are marketed, this technology could be developed into an off-the-shelf product, which could replace CAR T-cells
-
Pretargeting could prove that “we might only be at the tip of the iceberg”1 of immunotherapy
THE MARKET PLACE
The global immunotherapy drug market is projected to reach USD 201.52 Billion by 2021 from USD 108.41 Billion in 2016, growing at a CAGR of 13.5% during the forecast period of 2016 to 2021.
North America is expected to account for largest share in the immunotherapy drug market, by region in 2020 Asia-Pacific is expected to be the fastest-growing regional segment in the market.
STATUS
Our preliminary results demonstrate that the pretargeting treatment modality has an anti-leukemia effect in the BCL1 murine model. This is the first evidence that pretargeting is not just a theoretical prediction but also a feasible and doable technology.
GOAL
PRET’s primary aim is to perform the complete the animal studies with secured funding in order to demonstrate the proof of principle. Our secondary aim is to conclude a partnership to perform the complete achieve a proof of concept and further exploit wider application areas.
COLLABORATIONS
PRET Therapeutics collaborate with the Cancer Center of the Semmelweis University Budapest, Hungary, Biotherapy International, Weizmann Center Tel Aviv, Israel, The Institute of Microbiology of the Czech Academy of Sciences, EIT Health InnoStars and Biosystems International.