Summary
Lung cancer (LC), Breast cancer (BC), Colon Cancer (CC) are the 3 most prevalent cancer types with the highest death rates. While diagnostic imaging (e.g. CT) and tissue diagnostics (e.g. biopsies) are the most frequently used methods for diagnosing cancer, growing cancer incidence rates are calling for less invasive and cheaper methods of screening the global population. At the same time early detection through frequent screening could improve patient outcomes and save lives.
Bio-systems International (BSI) has developed an early stage detection In-Vitro Diagnostic (IVD) for lung cancer, what is patented, registered and ready to be launched. Further application areas for diabetes for the young (MODY), BC and CC are in the pipeline.
Lung cancer facts
LC mortality worldwide is at about 84%. While survival rates of other types of cancer have significantly improved over the same period, the five-year survival rate of LC improved only by 4-5% since 1975. Considering the frequency of lung cancer (e.g. 230.000 new cases per year in US) more people die each year of lung cancer than breast, prostate and colon cancer altogether.
Early diagnostics of LC could raise the five-year survival rate up to 50-60% compared to the current 16%, as it could detect advanced but largely asymptomatic cases among high risk persons (e.g. smokers) to which surgery with optional combination of chemotherapy could be applied in time.
BSI Pioneer technology
Generally, proteins can either be detected using antibodies, i.e. immunoassays or using mass spectrometry. According to recent studies and results immunoassays provide the user with higher reproducibility and are easier to translate and validate than mass spectrometry based techniques, as immunoassays are able to trace proteins both quantitatively and qualitatively. 1
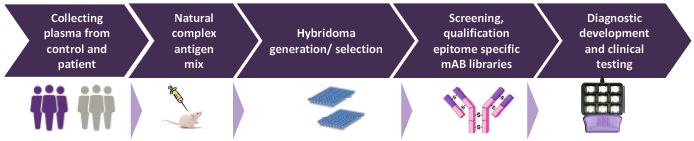
Key to proteomic diagnostic tool development is identifying differential expression of proteins related to a certain disease. Proteins in the blood change as a result of disease providing valuable information in relation to cancer development (including early stages). A protein signature is unique for a specific type of cancer. Based in this technology BSI has finalized its LC diagnostic tool. The test is performed from 7ml of blood of the patients and results are at hand within 48 hours. The product is based on a patented technology for profiling blood plasma proteome based on protected mAb technology.
Advantages of the system:
-
Easier to apply as IVD tools than many other forms of diagnostic and testing tools
-
Easy to collect samples from the blood
-
N o radiation exposer
-
Clinically validated
-
Fast results
-
CE mark